GS1 Healthcare Conference, 21-23 October 2014, Copenhagen, Denmark
The Power of GS1 Standards in Healthcare
Presentations - Poster Session - Photos - Programme - About the conference
Presentations
- Introduction to GS1, GS1 Healthcare Team
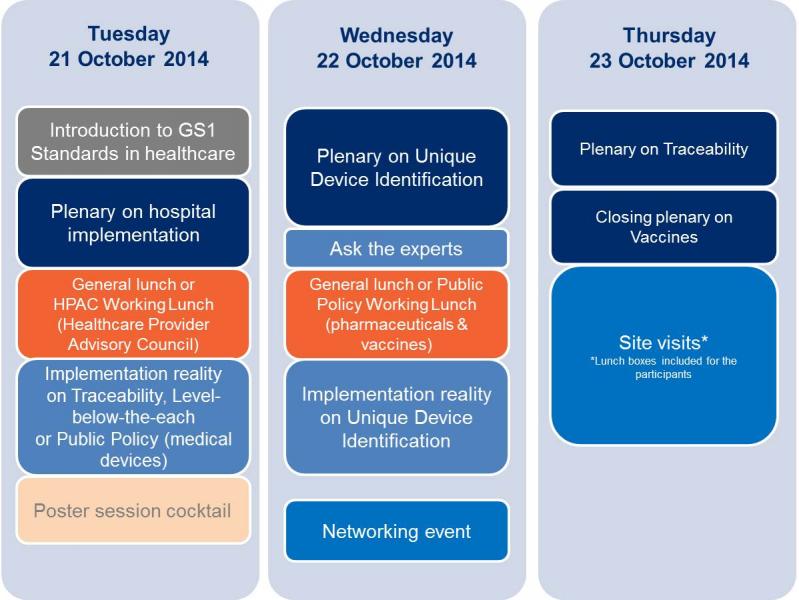
Day 1 - Tuesday 21 October
Plenary Session on Hospital Implementation
- Welcome to conference, Ulrike Kreysa and Miguel Lopera, GS1 Global Office
- The new NHS eProcurement strategy in the UK, Lord Philip Hunt, Healthcare Supply Association, Royal Society of Public Health
- The new NHS eProcurement strategy in the UK, Andy McMinn, Plymouth Hospital
- Reducing medication errors in Danish hospitals with primary package barcoding, Flemming Sonne, AMGROS
- How GS1 BarCodes improve logistics quality and patient safety, Viggo Nielsen, Hospital Pharmacy Capital Region
- Reducing medication errors in hospitals- the importance of bedside scanning, Richard Price, The European Association of Hospital Pharmacists (EAHP)
- Bedside scanning, the missing link in patient safety, Thomas De Rijdt, University Hospital Leuven
- How to implement traceability in a hospital? Erik Van Ark and Justin Bitter, Bernhoven Hospital
Implementation Reality Sessions
- Scanning at Point of Care - safer care for patients
- Traceability implementation for all stakeholders in the supply chain, from manufacturer to patient:
Day 2 - Wednesday 22 October
Plenary session on UDI
- Situation at IMDRF and European Union, Laurent Sellès, EU Commission
- A critical stage in the development of the EU legislation on UDI, Mike Kreuzer, Eucomed
- Past the first U.S. FDA UDI deadline, Jay Crowley, USDM
- UDI implementation challenges – focus on the UDI database, Volker Zeinar, B. Braun
- Australian Supply Chain reform, Kate Ebrill, NEHTA
- Impacts of the adoption of global standards in the Portuguese healthcare value chain, Prof Mateus
Ask the Experts Session
- Identification and marking of multi-country packages
- eCommerce harmonisation
- GS1 Healthcare intelligent package: the mobile app
Implementation reality sessions on UDI
- Medical devices: how to mark/identify my products
- GDSN implementation success stories and preparation for the UDI database
Day 3 - Thursday 23 October
- Key requirements of the EU Falsified Medicines Directive, Christoph Krähenbühl, 3C Integrity
- European Stakeholder Model (ESM) Verification of Medicinal Products in Europe, Mike Rose, Johnson &Johnson for EFPIA
- Implementation of serialisation & traceability at manufacturers - best practises, Angeline Riezebos, Sanquin Blood Supply, Divison Plasma Products
- Serialisation on a global level: is worldwide mass serialisation the long distance future? Mathieu Aman, F. Hoffmann-La Roche
- How to comply with the U.S. DSCSA rule using GS1 Standards, Chris Reed, Johnson & Johnson
- The road from Nordic Trade Item Numbers to Global Trade Item Numbers, Hans Andersson, LIF
- The WHO VPPAG recommendation for identification of vaccines and what it means for manufacturers - what are the opportunities, Rich Hollander, Pfizer
- The implementation of the WHO recommendations, UNICEF
- The proof of principle project in Tanzania, Henry Mwanyika, PATH
- The next global GS1 Healthcare conference in Latin America - invitation, Gerardo Brehm, GS1 Mexico
Poster sessions
- Amgros - Barriers to the use of barcode systems in the medication process
- GS1 Australia - Australia’s pharmaceutical wholesalers move to the global GS1 standards for their supply chain
- GS1 Denmark - Avoid problemes for pharmacy robots
- GS1 Finland - 10 Hospital Districts, 3 Data Carriers, 1 Standard
- GS1 France - GS1 applications in the Healthcare supply chain in France
- GS1 Germany - Management of Global Master Data using GDSN
- GS1 Hungary - GS1 Lib
- GS1 Ireland - Taking paper out of healthcare procurement
- GS1 U.S. - Unique Device Identification
- GS1 Global Office - 60 stakeholders endorse GS1 Standards
- GS1 Global Office - Meet our GS1 healthcare leadership team
- GS1 Global Office - Public Policy Database
Photos
Programme
Download the official programme
About the conference
Held twice per annum, the Global GS1 Healthcare Conference is a key event for sharing information to the GS1 Healthcare community. Healthcare leaders from private industry and government agencies will present the progress of worldwide efforts to implement GS1 Standards that improve patient safety, supply chain security and efficiency.
GS1 Healthcare community. Healthcare leaders from private industry and government agencies will present the progress of worldwide efforts to implement GS1 Standards that improve patient safety, supply chain security and efficiency.
Why should you join?
The three-day conference combines plenary sessions, implementation reality sessions – small work groups – working lunches, a networking event and gives the opportunity to attend site-visits of Danish stakeholders. The conference will take place a couple of weeks after the first U.S. FDA deadline which requires the unique identification of all class III medical devices sold in the U.S. Attend this conference and find out the latest news and first results of the U.S. FDA UDI, but also get the updates on UDI regulations from regulatory authorities and governments from around the world. Learn about different hospital implementations as well as healthcare traceability implementations, and hear how GS1 Standards can improve quality of care and reduce medication errors.
Free attendance for healthcare providers (hospital, hospital pharmacy...)
Fees
Full fee: 950€
Member of Global User Group (click here for list): 650€
Staff of an association: 650€
Hospital employee: Free
Regulatory body employee: Free







