Release 10.0, Ratified, Jun 2020
GS1 Healthcare GTIN Allocation Rules Standard
GTIN Allocation Rules for the Healthcare sector
Contents
- 1.1 Purpose and regulatory disclaimer
- 1.2 Scope
- 1.3 Guiding principles
- 1.4 Defining a new product compared to a pro...
- 1.5 GTIN non-reuse
- 2.1 New product introduction
- 2.2 Declared formulation or functionality
- 2.3 Declared net content
- 2.4 Dimensional or gross weight change
- 2.5 Add or remove a certification mark
- 2.6 Primary brand
- 2.7 Time critical or promotional product
- 2.8 Pack/case quantity
- 2.9 Pre-defined assortment
- 2.10 Price on pack
1 Introduction
The Global Trade Item Number® (GTIN®) provides a global standard by identifying any trade item upon which there is a need to retrieve predefined information and that may be priced, or ordered, or invoiced at any point in the supply chain.
The Healthcare GTIN Allocation Rules are designed to help industry make consistent decisions about how to manage the unique identification of trade items. These rules have been developed in accordance with the GS1 Global Standards Management Process (GSMP) and are considered a part of the GS1 system of standards. Overall costs are minimised while efficiencies and patient safety improve when all partners in the supply chain adhere to the Healthcare GTIN Allocation Rules.
The unique identification of trade items is critical to maintaining operational efficiencies that business partners rely on to exchange information about products in consistent ways, as well as ensuring the smooth operations of global supply chains.
Additionally, the unique identification of trade items is crucial when complying with various regulations across the globe. Finally, the communication of unique identification changes between trading partners is essential to ensure the right product is made available when needed.
![]() Note: The term ‘product’ as used throughout the GS1 Healthcare Allocation Rules refers to the trade items to which GTINs are assigned
Note: The term ‘product’ as used throughout the GS1 Healthcare Allocation Rules refers to the trade items to which GTINs are assigned
1.1 Purpose and regulatory disclaimer
1.2 Scope
1.3 Guiding principles
1.4 Defining a new product compared to a product change
1.5 GTIN non-reuse
This document aims at providing a globally harmonised framework for the implementation of the GS1 standards in order to improve supply chain efficiency and ensure patient safety.
![]() Important: The Healthcare GTIN Allocation Rules represents a minimum requirement. Please be advised that there may be regulation(s) in your market area that are more stringent and SHALL be adhered to. Refer to the Healthcare Public Policy Interactive Map for more information.
Important: The Healthcare GTIN Allocation Rules represents a minimum requirement. Please be advised that there may be regulation(s) in your market area that are more stringent and SHALL be adhered to. Refer to the Healthcare Public Policy Interactive Map for more information.
GS1 standards help improve supply chain operations, process efficiency, and comply with regulatory requirements to improve patient safety. This document provides clear rules for the allocation of the Global Trade Item Number (GTIN) to regulated healthcare products.
The rules outlined in this document are intended for the regulated healthcare sector. Some of the rules included are not applicable to other sectors and are not included in the GTIN Management Standard. Every effort has been made to harmonise rules that appear in both documents.
![]() Note: Additional terms are found in section 5 of this document and on the online glossary on the GS1 website and in the GS1 General Specifications.
Note: Additional terms are found in section 5 of this document and on the online glossary on the GS1 website and in the GS1 General Specifications.
![]() Note: Refer to the GS1 Healthcare website for general information.
Note: Refer to the GS1 Healthcare website for general information.
The following guiding principles should be considered by the brand owner when developing a GTIN assignment strategy for a new trade item and when introducing changes to an existing trade item.
■ Product Contained in Package: Is a stakeholder (e.g. care providers, consumers, patients, regulatory authorities and/or trading partners) expected to distinguish the changed or new product from previous/current products?
■ Label/Package: Is there a regulatory or liability requirement to disclose a change to the consumer and/or trading partner?
■ Label/Package: Is there a substantial change impacting the supply chain (e.g., how the trade item is shipped, stored, received or handled in the clinical setting)?
![]() Note: At least one of the guiding principles must apply for a GTIN change to be required.
Note: At least one of the guiding principles must apply for a GTIN change to be required.
A separate, unique GTIN is required whenever two products are different in any way that is relevant to the trading process, intended use, or point-of-care.
When making decisions about product identification, it is important to understand the differences between a NEW product and CHANGES to an existing product.
New products are those which do not currently exist in a brand owner’s product offering and are new to the marketplace. A new product should be considered an “addition” to an existing product offering. GS1 standards and the Healthcare GTIN Allocation Rules require that if a product is new, it should always be assigned a new GTIN to accurately distinguish the new product from those currently available in the marketplace or previously existing product that has been discontinued.
Changes to existing products are considered “replacement product” as determined by the brand owner. The Healthcare GTIN Allocation Rules define that a new GTIN is required when a change to certain attributes of an existing product change such that a new GTIN is required.
■ New product: A "new product" is defined as a product that does not currently exist or has not been available for sale and is an addition to the brand owner's portfolio/is new to the marketplace.
■ Product change: An existing product, currently in the brand owner's portfolio and available in the marketplace whose attributes have been changed.
An allocated GTIN SHALL NOT be reallocated to another trade item. Healthcare companies must ensure that GTINs allocated to regulated healthcare trade items SHALL never be reused.
Exception: Regulated healthcare trade items that have been withdrawn from the market and are reintroduced may use the original GTIN if they are reintroduced without any modifications or changes which require a new GTIN as specified by the Healthcare GTIN Allocation Rules or the GTIN Management Standard.
As an example: “Product A”, a first-generation injectable antibiotic, was withdrawn from the market by its manufacturer due to declining sales. After a 10-year absence from the market, “Product A” was reintroduced by the manufacturer, in its original form and package configuration, to treat infections resistant to newer antibiotics. In this example the original GTIN may be used.
![]() Note: GTINs assigned to regulated healthcare products have always been governed by a non-reuse policy. Outside of regulated healthcare, the general GTIN non-reuse rule went into effect on 1 January 2019 in response to digital business demand. GTINs discontinued and withdrawn from the market prior to 1 January 2019 may be considered for reuse one final time (*). However, companies are strongly advised to follow the non-reuse rule for all GTINs to avoid risks of conflicting data.
Note: GTINs assigned to regulated healthcare products have always been governed by a non-reuse policy. Outside of regulated healthcare, the general GTIN non-reuse rule went into effect on 1 January 2019 in response to digital business demand. GTINs discontinued and withdrawn from the market prior to 1 January 2019 may be considered for reuse one final time (*). However, companies are strongly advised to follow the non-reuse rule for all GTINs to avoid risks of conflicting data.
(*) If a GTIN was withdrawn prior to 1 January 2019, the previously applicable rules must be adhered to.
For more information refer to the GS1 General Specifications, GTIN Non-re-use section.
2 GTIN Allocation Rules
Although regulations are extremely important in this area, most non-regulated healthcare products follow broadly similar allocation rules to those in the general retail environment (see GTIN Management Standard). This document includes allocation rules needed in the regulated healthcare sector which are not found within the general retail environment.
The allocation rules in this section apply to any type of healthcare item.
Below are the rules that define when a GTIN SHALL be assigned (New Product), a changed (replacement) Product or an Equivalent product in order to be in conformance with the Healthcare GTIN Allocation Rules.
![]() Note: Equivalent – A product which can be substituted for the existing trade item based on supplier-defined functional equivalence to the trade item in a specific target market.
Note: Equivalent – A product which can be substituted for the existing trade item based on supplier-defined functional equivalence to the trade item in a specific target market.
■ For example, when the regulatory content of a product label differs in a way that impacts License or Registration in a particular market and limits distribution channels, it requires the product to be uniquely identified for supply chain purposes and regulatory control using a unique and separate GTIN.
■ Different markets may not impose the same level of restriction (e.g. License or Registration) which means both versions of the product, which are functionally equivalent, can be referenced as such in markets where the restriction is not imposed.
The Healthcare GTIN Allocation Rules are designed to help the healthcare industry make consistent decisions about the unique identification of trade items. This standard has been developed in accordance with the GS1 Global Standards Management Process (GSMP) and is considered a part of the GS1 system of standards.
Remember that all the Healthcare GTIN Allocation Rules and the three guiding principles need to be taken into consideration when making the final decision of whether to change a GTIN.
2.1 New product introduction
2.2 Declared formulation or functionality
2.3 Declared net content
2.4 Dimensional or gross weight change
2.5 Add or remove a certification mark
2.6 Primary brand
2.7 Time critical or promotional product
2.8 Pack/case quantity
2.9 Pre-defined assortment
2.10 Price on pack
A "new product" is defined as a product that does not currently exist or has not been available for sale and is an addition to the brand owner’s portfolio/is new to the marketplace.
Any new product requires the assignment of a new GTIN.
Relevant guiding principles:
| GTIN Rule Name | Is a care provider, consumer and/or trading partner expected to distinguish the new product from previous/current products? | Is there a regulatory/liability disclosure requirement to the consumer and/or trading partner? | Is there a substantial impact to the supply chain (e.g., how the product is shipped, stored, received, or handled in the clinical setting)? |
| New product introduction | YES | YES | YES |
2.1.1 Different language
This rule provides guidance on the allocation of GTINs to trade items regarding the addition and removal of languages based on the intended target market in which the product will be sold. This covers the language printed on the package itself as well as manuals or inserts that are considered part of the trade item.
Any change to language that impacts where a product can be sold or how trading partners and end users interact with it, requires the assignment of a new GTIN.
Hierarchy levels of GTIN assignment:
■ The GTIN is assigned at the hierarchy level in which the language is listed (i.e. the packaging level that contains the specific language).
■ A unique GTIN is assigned at every higher hierarchy level.
Example business scenarios that require a new GTIN:
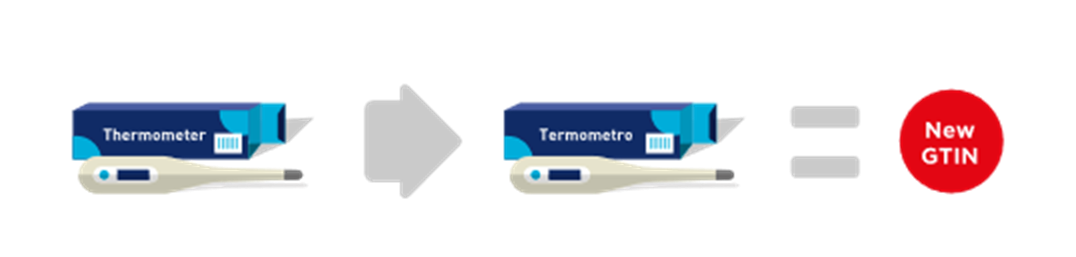
■ Single language products with different target market/country.
Two otherwise identical products - one targeted for an English-speaking country, the other for a Spanish-speaking country. As the two items exist in parallel and cannot be substituted (due to market acceptance and local labelling laws) a new language version to be sold in one target market/country requires a separate, unique GTIN than the other sold in a different target market/country.
Figure 2‑1 New product - new GTIN
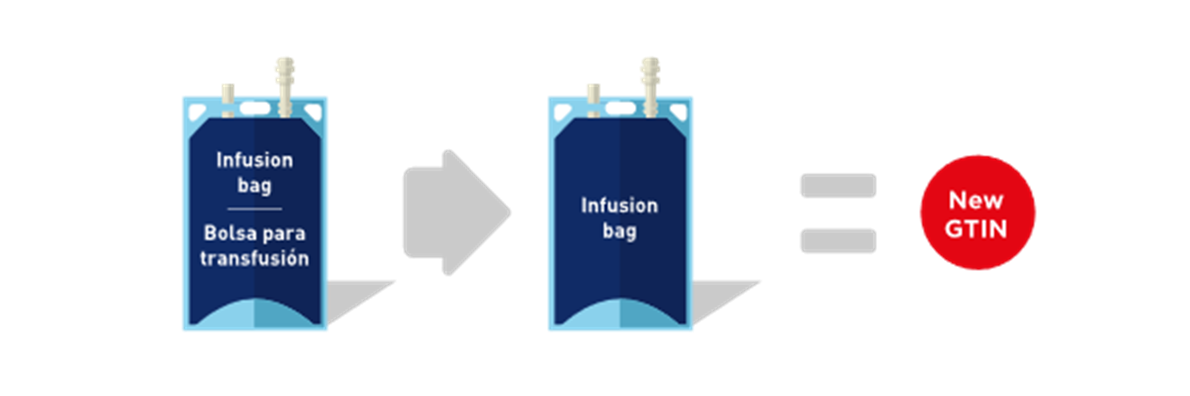
■ When a language is removed from a multilingual package, a new GTIN SHALL be assigned.
Figure 2‑2 Removal of a language from the package - new GTIN
In cases where there is more than one leaflet included in the product package, the rules above regarding removal and addition of languages apply.
Over-labelling for a specific target market
![]() Note: When additional labelling is added that does not conceal the previous information, a new GTIN is not needed.
Note: When additional labelling is added that does not conceal the previous information, a new GTIN is not needed.
Example of minor artwork modification where a new GTIN in not needed:
Minor artwork or other minor modifications to packaging, which are not relevant to trading partners because they do not impact the information concerning the exchange of products, do not require the allocation of different GTINs.
Figure 2‑3 Minor updates to packaging - same GTIN

Additional language on the packaging sold in several markets
Unlike the single language packaging, many products are packed for multiple countries and markets. When there is an additional language added to a trade item with an existing language, the GTIN remains the same.
Figure 2‑4 Addition of a language to an existing package - same GTIN

Additional information:
■ Consider applicable target market regulations related to language requirements in which the product will be sold.
■ Some target markets require more than one language, consult local regulations.
2.1.2 Assignment of GTINs within a trade item hierarchy
Information on marking (e.g. using barcodes) for Regulated Healthcare Trade Items is covered in the section titled Healthcare Secondary Packaging (Regulated Healthcare Retail Consumer Trade Items) of the GS1 General Specifications. For additional implementation information refer to the GS1 AIDC Healthcare Implementation Guideline.
Unit of Use / Single Unit
Products in most sectors are identified at multiple packaging hierarchy levels (see section 2.8). The lowest hierarchy level of trade items within the GS1 System is traditionally referred to as the “each” level. The “each” level trade item may contain more than one unit of use. In this case there may be the need to identify levels below the ‘each’ down to the single unit or unit of use. For the purpose of this rule, ‘single unit’ and ‘unit of use’ are synonymous.
![]() Note: Depending on the target market, labelling requirements for the level below the each and single unit might apply.
Note: Depending on the target market, labelling requirements for the level below the each and single unit might apply.
Hierarchy levels of GTIN assignment:
■ A GTIN is assigned at the “each” level.
■ Higher levels of packaging are assigned a separate, unique GTIN at each level if these levels are considered trade items. See section 2.8 for more information.
■ Levels below the ‘each’, down to the unit of use / single unit should have a GTIN assigned. However, it may or may not be marked on those levels.
Example business scenarios that require a GTIN:
Medical Devices
It is recommended for medical devices that there is only a single level of a product below the lowest packaged level to ensure accuracy in supply chain and ensure traceability.
![]() Note: However, it is acknowledged that a small number of exceptions may exist where more than one such level exists.
Note: However, it is acknowledged that a small number of exceptions may exist where more than one such level exists.
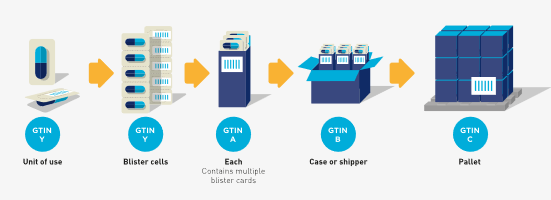
In the following example the ‘each’ contains a count of two (i.e., for illustration purposes, the count could mean two devices). In this case there are two single units, or ‘units of use’, per ‘each’.
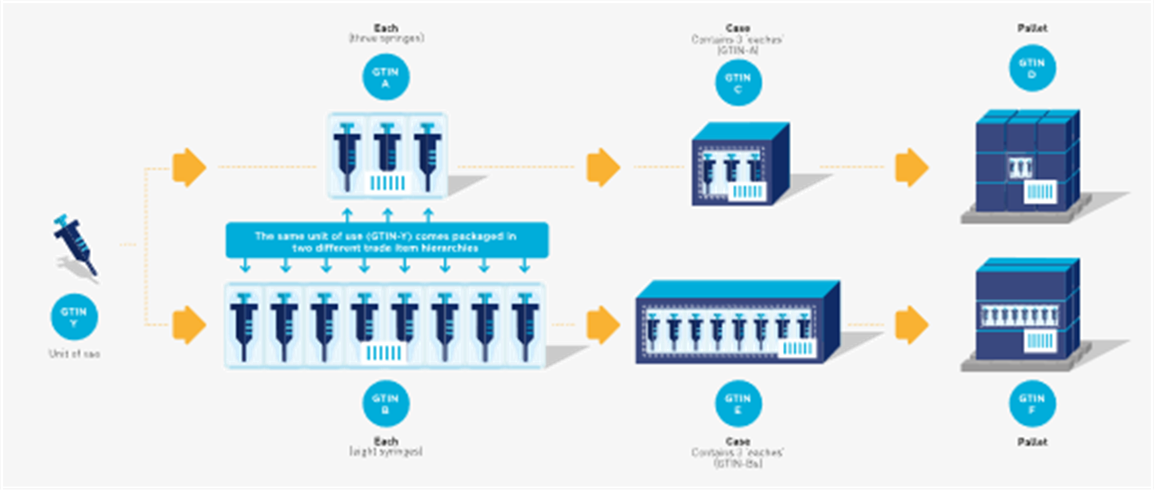
Figure 2‑5 Hierarchy with a unit of use
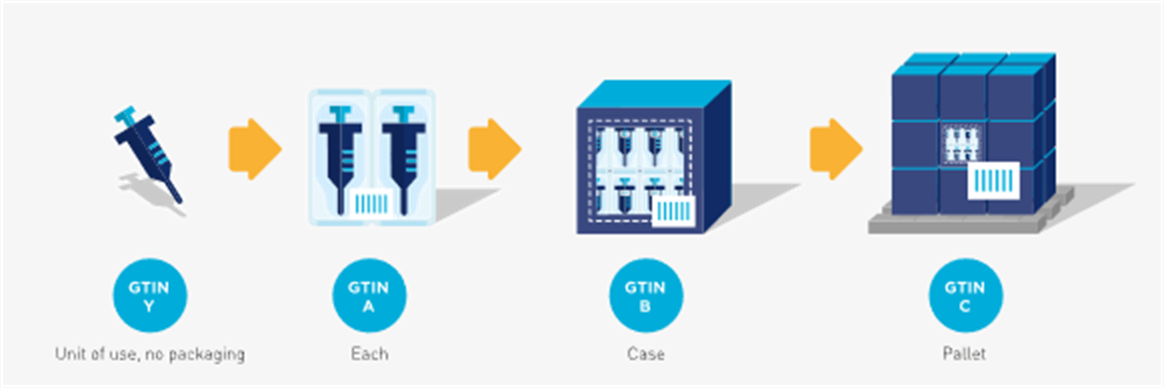
In the example a unit of use device is available in two configurations, a saleable item (i.e. the ‘each’) with three syringes and a saleable item with eight syringes. In both cases the unit of use would have the same GTIN while the rest of the packaging hierarchy is assigned different and unique GTINs.
Figure 2‑6 In this example, the unit of use (GTIN-Y) is packaged in two different trade item hierarchies but may or may not be marked
Electrode dots are used in electrocardiograms. The lowest traded / packaged unit is a ‘Bag’ containing 3, 5 or 10 ‘strips’ where each ‘strip’ contains 5 or 10 individual dots. The bag is the lowest traded unit (lowest level tracked in the supply chain) and are often packaged at higher levels of 5 or 10 bags to an intermediate packaging level. There may be 4 or 6 of these in a shipper case (i.e. logistics unit). The strips and dots are not individually packaged.
In this example the dots are the unit of use. There may be no set number used in each procedure, however, they have set locations on a patient. The reason for the variation is that sometimes dots fall off and a new one used. A single strip may have dots removed and used on multiple patients, they may be removed from a store area or trolley and the unused one may be returned to storage.
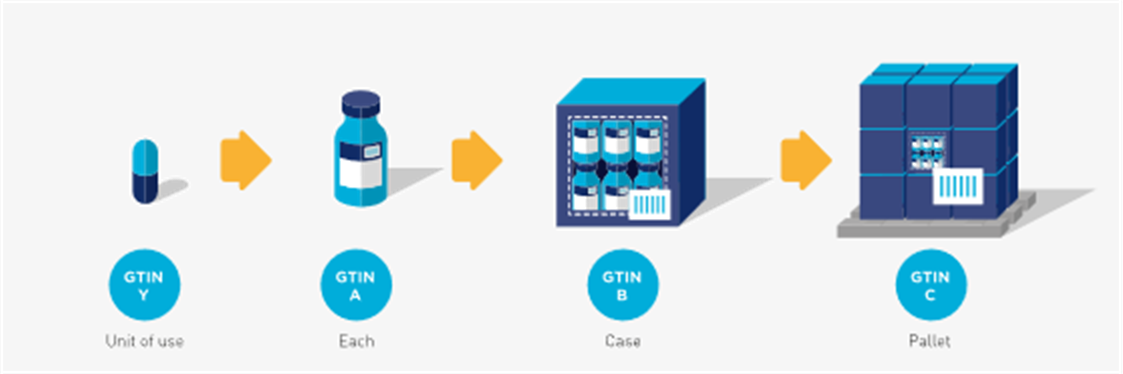
In the examples in figures 2-6 and 2-7 the ‘each’, ‘case’, and ‘pallet’ are trade items and are identified with separate, unique GTINs (A, B, and C respectively). The ‘level below the each’ contains a single unit (i.e. unit of use) and should have a GTIN assigned (GTIN Y). However, it may or may not be marked (i.e. barcode/HRI or non-HRI) on the single unit.
Figure 2‑7 Hierarchy with a unit of use (glucose test strip example)
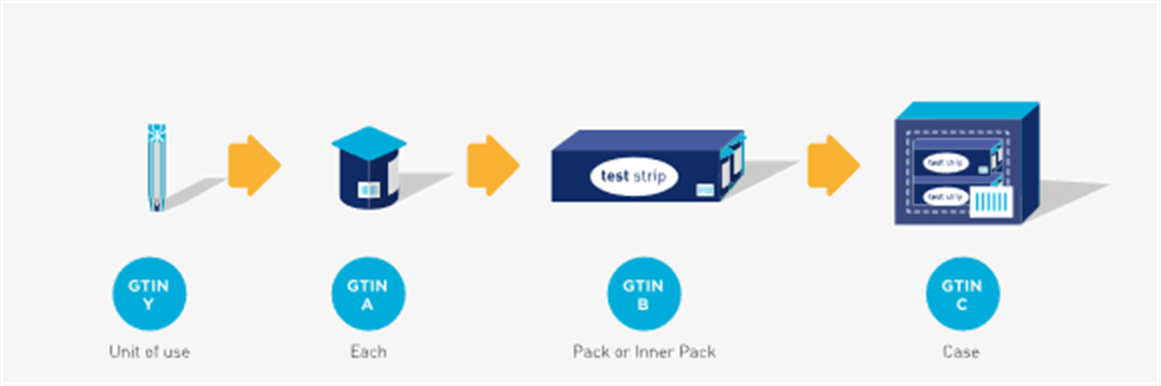
Figure 2‑8 Hierarchy with a single unit
Single unpackaged pills/tablets/capsules/caplets and those packaged in blister cells
GTINs allocated to single unpacked pills, tablets, capsules, or caplets are not expected to be marked using AIDC technology (e.g., barcodes). If the ‘each’ contains several units of the product that can be easily separated into individual units of use, as in a perforated blister package, the individual units should be identified, but may or may not be marked.
Figure 2‑9 In this example the blister card is identified and marked with a single GTIN. The unit of use is identified with a GTIN but is not marked. The marking is at the discretion of the brand owner
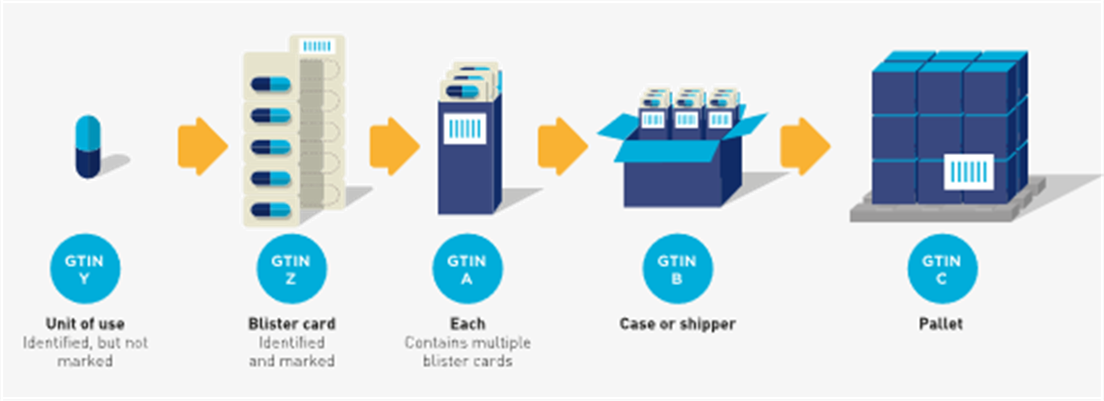
Figure 2‑10 In this example each blister cell is identified and marked. The blister card is not identified or marked. The marking is at the discretion of the brand owner
Additional information:
Unpackaged solutions/liquids/creams/gels/powders/aerosols
A GTIN is not expected to be allocated to unpackaged liquids, creams, gels, powders, and aerosols for example, unless required by regulation or as agreed within a trading partner relationship. GTINs assigned to these unpackaged items are not marked using AIDC technology (e.g., barcodes).
![]() Note: Higher levels of packaging, such as a case or pallet, can be identified with a GTIN when it is identifying a trade item. If trade items were grouped for the purpose of transport and/or storage, the grouping would be classified as a logistics unit and identified with a Serial Shipping Container Code (SSCC). A case, pallet or other trade item grouping can be assigned both a GTIN for product identification and SSCC for logistics purpose. For more information on SSCC refer to the GS1 General Specifications.
Note: Higher levels of packaging, such as a case or pallet, can be identified with a GTIN when it is identifying a trade item. If trade items were grouped for the purpose of transport and/or storage, the grouping would be classified as a logistics unit and identified with a Serial Shipping Container Code (SSCC). A case, pallet or other trade item grouping can be assigned both a GTIN for product identification and SSCC for logistics purpose. For more information on SSCC refer to the GS1 General Specifications.
Differentiation between primary package and secondary packages in a one to one (1:1) relationship
Some healthcare processes require the capability to clearly differentiate between a healthcare trade item in its primary and secondary packaging, even if they share a “one to one” (1:1) relationship. An example could be a tube of cream in a box, a vial in a box or a syringe in a unit carton. In this situation the trade item primary package and secondary package may have different GTINs assigned when required by regulation or as agreed within a trading partner relationship in the absence of regulatory requirements. GTIN allocation and the marking of GTINs is made at the discretion of the brand owner.
Refer to the GS1 General Specifications for further information on trade item groupings.
Refer to section Healthcare Primary Packaging (Non-Retail Trade Items) of the GS1 General Specifications for more information.
2.1.3 Single-use non-sterile devices/multiple devices never sold separately
Single use, non-sterile, medical devices packaged more than one to a package and multiple devices not commonly sold separately (e.g., number of cotton swabs, individually unpackaged, and contained in a single bag) may require a unique GTIN to be assigned.
Hierarchy levels of GTIN assignment:
■ A GTIN is assigned at the ‘each’ level.
■ Higher levels of packaging are assigned a separate, unique GTIN at each level if these levels are considered trade items. See section 2.8 for more information.
■ Level below the each, down to the single unit (i.e., unit of use) may have a GTIN assigned. However, it may or may not be marked on those levels.
Example business scenarios that require a new GTIN:
Examples of multiple devices not commonly sold separately that may require assignment of a GTIN such as high quantity screws/pins, gloves/gowns, swabs, tape, number of cotton swabs, individually unpackaged, and contained in a single bag and examples of single-use non-sterile devices include gauze, swab, tissue, etc.
Additional information:
For information on multi-use non-sterile devices see section 2.1.4.
2.1.4 Multi-use non-sterile devices
A GTIN should be allocated to a single unit of a multi-use non-sterile device.
Hierarchy levels of GTIN assignment:
■ The GTIN is assigned at the single unit.
■ A separate GTIN is assigned at each packaging level of the hierarchy which may be priced, ordered, or invoiced.
Example business scenario:
A non-disposable blood pressure cuff is one example of a multi-use non-sterile device.
Additional information:
GTINs, assigned to the level below the each (e.g. single unit, or unit of use), may be marked using AIDC technology (e.g., barcodes). Depending on the type of device there may be regulatory requirements for direct part marking.
2.1.5 Barrier packs – sealing inner through outer (SITO process)
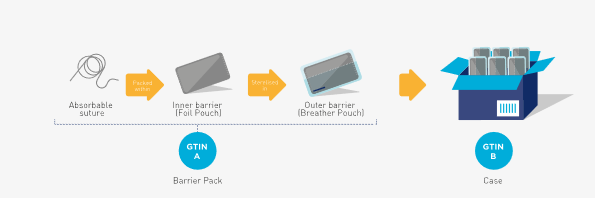
The general rule is that each packaging level requires a separate, unique GTIN. However, for certain manufacturing processes, such as the formation of a sterile double barrier package, where one barrier cannot be created as sterile without the other is a unique manufacturing situation and is therefore considered an integral part of a single package level when complete. The independent and separate constituent barrier levels post manufacturing process are not considered as distinct packaging levels of the trade item hierarchy for GTIN assignment, provided the higher level (e.g., outer barrier) only contains one unit of the lower level (e.g., inner barrier) and the inner barrier requires the outer barrier to form the sterile unit within the manufacturing process. See Figure 2‑11.
The example below shows a typical product where the sterilisation requires two packaging levels (i.e. double barrier packaging). When the suture is used, certain packaging levels may only be opened in a sterile environment. However, the foil pouch itself or the suture do not require a separate GTIN. Note that in this example, the breather pouch contains one foil pouch, which itself contains one suture – this is a precondition for allocating the same GTIN to this trade item. When the foil pouch contains more than one unit of use, this invokes the unit of use rule as per section 2.1.2 for the assignment of a GTIN.
Figure 2‑11 Barrier packs (sterile packaging)
“Functionality” is defined as the particular use or set of uses for which something is designed. “Formulation” is defined as a list of the ingredients or components used to create a trade item.
A change to the formulation or functionality that affects the legally required declared information on the packaging of a product only requires the assignment of a new GTIN if the brand owner expects the customer or supply chain partner to distinguish the difference between the products prior and after the change. Both conditions must be met requiring the assignment of a new GTIN.
Example business scenarios that require new GTIN
■ Change to an active ingredient in a product
■ Change to an excipient in a product.
■ Change to a different material for the primary packaging.
■ The addition of a new assay to laboratory testing equipment.
Relevant guiding principles:
| GTIN Rule name | Is a consumer, patient, care provider and/or trading partner expected to distinguish the changed or new product from previous/current products? | Is there a regulatory/liability disclosure requirement to the consumer and/or trading partner? | Is there a substantial impact to the supply chain and trading partners (e.g., how the product is shipped, stored, received, or handled in the clinical setting)? |
| Declared Formulation or functionality | YES | YES | YES |
2.2.1 Patient specific product
In the case where a product is prepared specifically for an individual patient (for example in a hospital pharmacy or by an implant manufacturer) the party preparing or manufacturing the product is responsible for assigning the GTIN. A patient specific product should be identified so that it is uniquely attributed to the individual patient and/or a specific production instance.
Hierarchy levels of GTIN assignment:
■ A GTIN is assigned to the base product defined by key properties like ingredients, basic formula, indications, fundamental design etc. The patient specific product is then identified by the GTIN for the base product and a batch /lot and/or serial number. A new GTIN shall be assigned when any of the key properties of the base product changes.
![]() Note: At the discretion of the brand owner a new GTIN may be assigned to each patient specific product. This method is limited by the number of GTINs available to the manufacturer or dispenser.
Note: At the discretion of the brand owner a new GTIN may be assigned to each patient specific product. This method is limited by the number of GTINs available to the manufacturer or dispenser.
Example business scenario:
A hospital pharmacy prepares a specific product designed for a specific patient. In some cases, this is referred to a ‘personalised medicine”.
See section 2.2.2 for example of configurable medical devices.
2.2.2 Configurable medical devices
A configurable medical device is a product that consists of multiple components, some of which may be selected by the customer based on a list provided by the manufacturer. The possible configurations are determined by product design. In all cases, configurable medical devices are considered to be and intended to be used as, a single trade item.
Hierarchy levels of GTIN assignment:
■ A GTIN is assigned to the entire configurable medical device.
□ OPTION 1: Assign a GTIN to every final instance of the device. For example, each customised configuration has its own GTIN.
□ OPTION 2: Assign a GTIN to the base component, (i.e. that portion of the device to which optional components are added). For example, the base bed frame/chassis, the base monitor stand, etc. Then assign a serial number to the final customised instance of the device upon completion. Changes, modification or maintenance to the device could then be tracked via changes, modifications or maintenance logs at the serial number level.
■ A change in form, fit, or function to a mandatory component affecting intended use, requires a change to the GTIN and/or GTIN plus serial number of the entire configurable medical device. Mandatory components are those that are required to deliver the functionality of the device. Changes to or the removal of, a mandatory component, impacting device form, fit, or function, require a GTIN change.
■ Changes to optional components impacting device form, fit, or function, require a GTIN change. Similarly, removal of optional components from the set of available components requires a GTIN change.
Exemptions:
![]() Note 1: The addition of new components (that do not alter form, fit, or function affecting intended use) to a mandatory component selection list does not require a GTIN change.
Note 1: The addition of new components (that do not alter form, fit, or function affecting intended use) to a mandatory component selection list does not require a GTIN change.
![]() Note 2: The replacement of an optional component with a functionally equivalent component does not require a new GTIN.
Note 2: The replacement of an optional component with a functionally equivalent component does not require a new GTIN.
![]() Note 3: The addition of new optional components not affecting form, fit, or function to those available for the configurable medical device does not require a GTIN change.
Note 3: The addition of new optional components not affecting form, fit, or function to those available for the configurable medical device does not require a GTIN change.
Example business scenarios that require GTIN change:
Components, and how they are designed to interact with each other, establish the identification requirements for the complete device. A configurable medical device is identified by its GTIN and applicable variable data attributes (e.g., batch or lot, serial number, expiration date, production date, etc.), thus enabling configurations of the medical device to vary by combinations of components, while maintaining the same GTIN except as noted below.
Figure 2‑12 Example of a configurable medical device
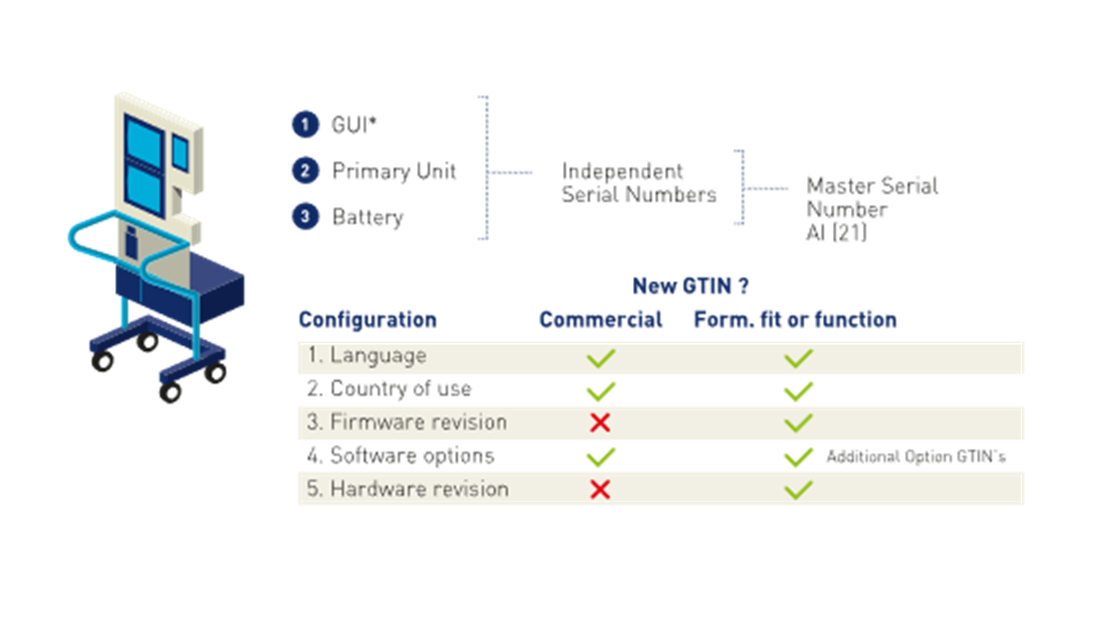
*GUI = Graphical User Interface
■ In the case where these products are prepared specifically for a patient, normal GTIN allocation rules may not be applicable. For these specialised devices, the device should be uniquely identified and marked.
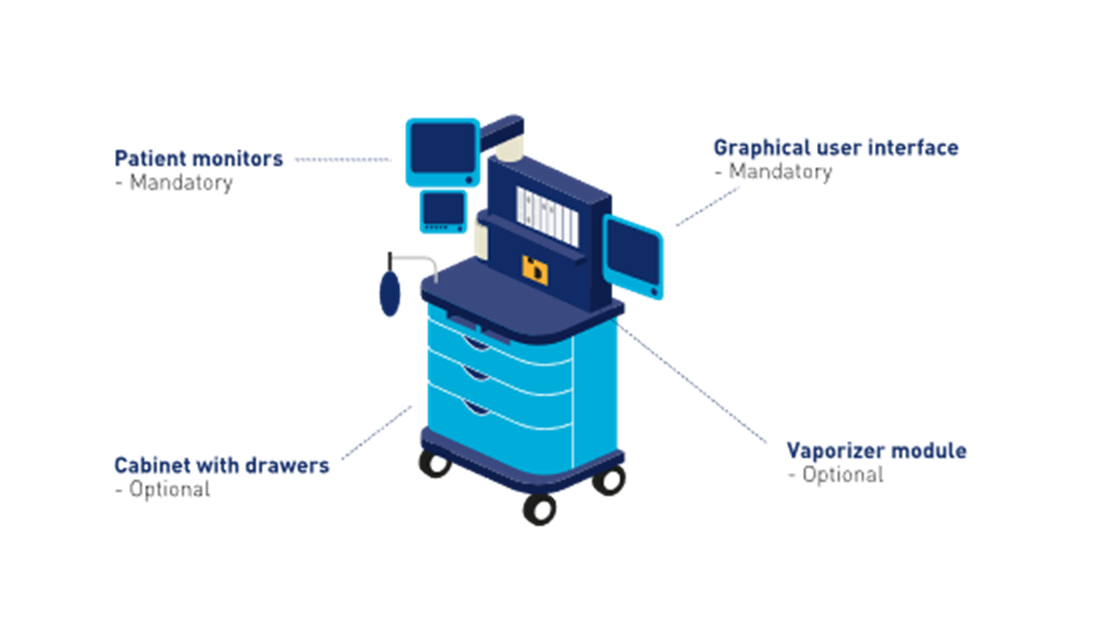
■ Configurable medical devices may also include optional components, which may be included in a configuration of the medical device. Optional components provide features or extensions to functions.
The configurable medical device example below includes the following components for purposes of illustrating mandatory and optional components.
■ Graphical user interface (GUI) – mandatory component
■ Patient monitors – mandatory component
■ Cabinet with drawers – optional component
■ Vaporizer module – optional component
Figure 2‑13 Example of a configurable medical device
Additional information:
When changes are made to a configurable medical device, a Global Individual Asset Identifier (GIAI) could be assigned by the device owner (this could be the manufacturer for leased or consigned devices or the end user for owned devices) and management of changes to the device would be handled via a change or maintenance record associated with the specific unit identified by a GIAI. For more information about the GIAI key refer to the Assets section of the GS1 General Specifications.
2.2.3 Software as a medical device
Medical device software is a software system developed for the purpose of being incorporated into a medical device or is intended itself for use as a medical device. Software within the scope of these rules is a trade item and is priced, ordered, or invoiced.
Medical device software may be structured similar to configurable medical devices, including mandatory and optional features, which are similar to device components, see section 2.2.2.
A major change in medical device software functionality affecting, form, fit, or function and intended use, requires a GTIN change.
Hierarchy levels of GTIN assignment:
■ A GTIN is assigned to the medical device software which can be ordered, invoiced or shipped. Different licensing levels (e.g., limited number of users versus enterprise licenses) require assignment of a different GTIN.
![]() Note: Examples of major changes include new or modified algorithms, database structures, architecture, new user interfaces, or new channels for interoperability.
Note: Examples of major changes include new or modified algorithms, database structures, architecture, new user interfaces, or new channels for interoperability.
![]() Note: Changes to software occur throughout the life of the device. For medical device software, minor changes shall not require a new GTIN. Examples of minor changes include bug fixes, aesthetics, usability enhancements, security patches, or operating efficiency. Application identifier (8012) for Software version can be used when it is necessary to manage software versions. Software versioning is the process of assigning unique version numbers to unique states of computer software. The use of application identifier (8012) for Software version shall occur in combination with the GTIN. For more information regarding software versioning, refer to the GS1 General Specifications, section titled Software version: AI (8012).
Note: Changes to software occur throughout the life of the device. For medical device software, minor changes shall not require a new GTIN. Examples of minor changes include bug fixes, aesthetics, usability enhancements, security patches, or operating efficiency. Application identifier (8012) for Software version can be used when it is necessary to manage software versions. Software versioning is the process of assigning unique version numbers to unique states of computer software. The use of application identifier (8012) for Software version shall occur in combination with the GTIN. For more information regarding software versioning, refer to the GS1 General Specifications, section titled Software version: AI (8012).
Example business scenario that require GTIN change:
■ Once installed, medical device software SHALL be identifiable with its assigned GTIN when separated from its packaging or physical documentation.
Example business scenario that does not require GTIN change:
■ The example in section 2.2.2 includes medical device software that operates the device. This software may be configured based on selected software features and device components. For example, if an additional patient monitor is selected as an optional component, the software must be configured to enable this component (patient monitor). In such cases, the GTIN assigned to the software does not require a GTIN change.
Additional information:
Medical device software that is distributed using a physical medium SHALL be identified with the same GTIN on the physical medium as that assigned to the software. Medical device software that is distributed virtually such as via a download SHALL have the GTIN and any relevant application identifiers displayed within the software such as on the “About” screen.
“Net content” is defined as the amount of the consumable product of the trade item contained in a package, as declared on the label, which may include net weight, volume, count, units.
Any change (increase or decrease) to the legally required declared net content that is printed on the pack, requires a GTIN change.
Hierarchy levels of GTIN assignment:
■ The level at which the net content change occurs requires a new GTIN and all higher levels of the hierarchy impacted shall have a new GTIN.
■ If the count at the base unit level, or the level below the ‘each’ changes a new GTIN SHALL be assigned.
Example business scenarios that require GTIN change:
Figure 2‑14 Change in declared net content - new GTIN

![]() Note: Information systems need to distinguish between old and new healthcare items where there is a declared change in net content. Failure to distinguish old and new could lead to medical error and/or inaccurate unit pricing.
Note: Information systems need to distinguish between old and new healthcare items where there is a declared change in net content. Failure to distinguish old and new could lead to medical error and/or inaccurate unit pricing.
Additional information:
Declared net content is what is used to develop shelf labelling and price per unit declared to the consumer. Declared net content is also important within clinical environments such as pharmacy. Accuracy of data and the ability to distinguish between products on the basis of net content is essential and failure to comply may result in a penalty or risk to patients/consumers.
Relevant guiding principles:
| GTIN Rule name | Is a consumer, patient, care provider and/or trading partner expected to distinguish the changed or new product from previous/current products? | Is there a regulatory/liability disclosure requirement to the consumer and/or trading partner? | Is there a substantial impact to the supply chain and trading partners (e.g., how the product is shipped, stored, received, or handled in the clinical setting)? |
| Declared net content | YES | YES | YES |
A change of over 20% to a physical dimension, on any axis (e.g., height, width, depth), or gross weight, requires assignment of a new GTIN.
![]() Note: Changes below 20% may require a new GTIN at the discretion of the brand owner.
Note: Changes below 20% may require a new GTIN at the discretion of the brand owner.
Hierarchy levels of GTIN assignment:
■ The GTIN assignment occurs at the trade item or base unit level.
■ A unique GTIN is assigned at every existing level of the packaging hierarchy above the trade item/base unit level.
Example business scenarios that require GTIN change:
■ The gross weight of a product increases by 50% from 0.34 kg (0.75 lb) to 0.51 kg (1.125 lb) due to a change in the packaging material.
■ A case or pallet orientation (there is no change to the trade item count) may be changed such that one or more axis changes.
■ In order to reduce the variety of folding box formats, a folding box with the dimensions of 47 x 18 x 127 mm is changed to 62 x 20 x 115 mm.
Figure 2‑15 Dimension or gross weight change
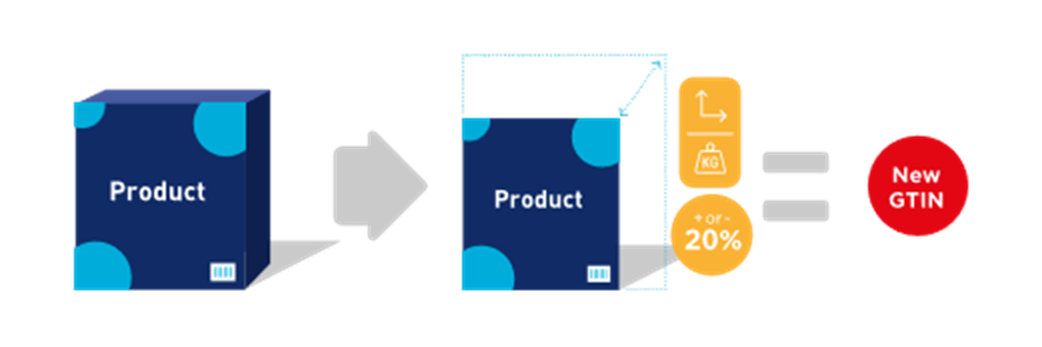
![]() Note: The 20% change applies to each individual axis and not cube/volume.
Note: The 20% change applies to each individual axis and not cube/volume.
Additional information:
■ This part of the standard only applies to changes to the dimensions and the gross weight of a product. Any change to declared net content is governed by the rule on declared net content in section 2.3.
■ Cumulative changes in avoidance of the 20% threshold, without changing the GTIN, is an unacceptable practice. Trading partners should be notified about all dimensional changes. Cumulative changes might cause problems for trading partners and may obstruct the flow through of products.
■ Refer to the GS1 Package Measurement Rules Standard for a consistent, repeatable process to determine measurements for a given product.
Relevant guiding principles:
| GTIN Rule Name | Is a care provider, consumer and/or trading partner expected to distinguish the changed or new product from previous/current products? | Is there a regulatory/liability disclosure requirement to the consumer and/or trading partner? | Is there a substantial impact to the supply chain (e.g., how the product is shipped, stored, received, or handled in the clinical setting)? |
| Changes in dimensions | YES | NO | YES |
Within the healthcare sector there are many examples of certification marks. A certification mark is a symbol, logo or wording on a product that declares conformance to a regulated set of criteria (e.g., European Certification Mark CE). When a product is changed to include a certification mark (which was not previously shown on the packaging or product itself) a new GTIN should be allocated for markets where the certification mark is of relevance. It is a key principle of GTIN Allocation that the GTIN uniquely identifies the product and its packaging configuration.
A change to packaging to add a new, or remove an existing certification mark (e.g., European Certification Mark CE), that has significance to regulatory bodies, trading partners or to the end consumer, requires assignment of a new GTIN.
Hierarchy levels of GTIN assignment:
■ The GTIN change occurs at the base unit level.
■ A unique GTIN is assigned at every existing level of the packaging hierarchy above the base unit level.
Example business scenarios that require GTIN change:
Certification Marks that appear, or change, on product labels that impact global distribution channels due to country licence or registration, must be communicated between trading partners and therefore require a GTIN change.
Figure 2‑16 Inclusion of a Certification Mark – new GTIN

However, it should also be noted that when a certification mark is added to enable sales in a new country/market it has no impact on countries/markets where the product was previously sold – in this case there is no need to allocate a new GTIN in the scenario above.
Additional information:
Brand owners are responsible for internal control of their inventory and any return systems. It is important that such systems, as well as phase-in & phase-out logistic management, can distinguish between ‘old’ and ‘new’ product. When this can be effectively achieved, for example using the batch number or product variant, there is no need to allocate a new GTIN in this scenario, if the external supply chain is unaffected.
![]() Note: Be aware of target market, regulatory and customer requirements if this is an implemented practice.
Note: Be aware of target market, regulatory and customer requirements if this is an implemented practice.
Relevant guiding principles:
| GTIN Rule name | Is a consumer, patient, care provider and/or trading partner expected to distinguish the changed or new product from previous/current products? | Is there a regulatory/liability disclosure requirement to the consumer and/or trading partner? | Is there a substantial impact to the supply chain and trading partners (e.g., how the product is shipped, stored, received, or handled in the clinical setting)? |
| Add or remove certification mark | YES | YES | YES |
The primary brand is the brand most recognisable by the care provider or patient, as determined by the brand owner, and can be expressed as a logo and/or words, registration mark or trademark.
A change to the primary brand that appears on the trade item, requires assignment of a new GTIN.
Hierarchy levels of GTIN assignment:
■ The GTIN change occurs at the trade item, base unit level or level below the each if appropriate.
■ A unique GTIN is assigned at every existing level of the packaging hierarchy above the trade item/base unit level.
Example business scenarios that require GTIN change:
The company’s primary brand name changed from “Healthcare Products Company” to “Leading Edge Healthcare Medical Products”.
Additional information:
Co-branding: The act of applying a second brand (the ‘co-brand’) by a company under contractual agreement with the original brand owner.
■ The company owning the co-brand is responsible for GTIN allocation.
■ The co-brand being applied shall be constructed as the prominent brand on the package as viewed by the customer, thus ensuring relationship of the product with the co-brand, acknowledged as the ‘Primary Brand’ of the co-branded product.
![]() Note: Contractual relationships may dictate that the ‘Primary Brand’ is not that of the co-brand, therefore the first and original brand shall remain prominent on the package. In this case, responsibility for GTIN allocation remains with the original brand owner
Note: Contractual relationships may dictate that the ‘Primary Brand’ is not that of the co-brand, therefore the first and original brand shall remain prominent on the package. In this case, responsibility for GTIN allocation remains with the original brand owner
Distributed by: Products for which an agreement exists between the Brand Owner and the party identified on the label as the Distributor. The Brand Owner remains responsible for GTIN assignment and therefore no new GTIN is necessary when the Distributed by party identification is added to the label.
![]() Note: The ‘Distributed by’ party identification must not include any registration marks or trademarks and must be made in plain text only.
Note: The ‘Distributed by’ party identification must not include any registration marks or trademarks and must be made in plain text only.
Own Brand Label: Products wherein an agreement exists between the Original Manufacturer and the party identified on the label as the Brand Owner. The Brand Owner takes responsibility for GTIN assignment and therefore retains the alignment of brand to GTIN allocation.
Relevant guiding principles:
| GTIN Rule name | Is a consumer, patient, care provider and/or trading partner expected to distinguish the changed or new product from previous/current products? | Is there a regulatory/liability disclosure requirement to the consumer and/or trading partner? | Is there a substantial impact to the supply chain and trading partners (e.g., how the product is shipped, stored, received, or handled in the clinical setting)? |
| Primary brand | YES | YES | NO |
Promotions are normally short-term modifications to the way the item is presented.
A change to a product that is being promoted (including packaging changes) for a specific event or date, impacting the required handling in the supply chain to ensure the trade item is available for sale during a specified time period, requires assignment of a new GTIN.
Hierarchy levels of GTIN assignment:
■ No GTIN change is required at the base unit level.
■ Existing levels of the packaging hierarchy above the base unit require a unique GTIN to be assigned for time critical promotions.
Example business scenarios where a unique GTIN at higher level packaging (e.g., pack, case, pallet) are required:
■ A free trial item (not identified with its own GTIN) is attached to an existing item for a promotional period, the declared net content of the original item is unchanged and packaging dimensions and the gross weight of the product are NOT changed by more than 20%.
Example business scenarios that do not require GTIN change:
■ Promotion: buy 2, get 1 free.
■ The graphics on bandages rotate quarterly. The graphics have no seasonal or time critical relevancy and are considered flow-through products.
![]() Note: Any promotion impacting the content of the product, or requiring a new regulatory filing, is considered a major change and a new GTIN must be assigned.
Note: Any promotion impacting the content of the product, or requiring a new regulatory filing, is considered a major change and a new GTIN must be assigned.
Additional information:
■ For time critical or promotional products, the GTIN for the trade item/base unit level does not need to be changed, but for tracking in the supply chain, higher levels of packaging need to be uniquely identified.
■ Local, national or regional regulations may require more frequent GTIN changes. Such regulations have precedence over the rules provided within the healthcare GTIN Allocation Rules.
Relevant guiding principles:
| GTIN Rule name | Is a consumer, patient, care provider and/or trading partner expected to distinguish the changed or new product from previous/current products? | Is there a regulatory/liability disclosure requirement to the consumer and/or trading partner? | Is there a substantial impact to the supply chain and trading partners (e.g., how the product is shipped, stored, received, or handled in the clinical setting)? |
| Time critical or promotional product | YES | NO | YES |
Hierarchy levels of GTIN assignment:
■ A unique GTIN is assigned at every existing level of the hierarchy including and above the lowest level that is changed.
Example business scenarios where a unique GTIN at the higher level packaging (e.g., pack, case, pallet) are required:
■ A case configuration changes from containing 8 trade items to containing 12 trade items, the case needs to be uniquely identified.
■ A pallet configuration changes from containing 12 cases to containing 16 cases, the pallet needs to be uniquely identified.
Refer to section 2.1.2 for assignment of GTINs to the each, level below the each, single unit and higher levels of packaging.
Relevant guiding principles:
| GTIN Rule name | Is a consumer, patient, care provider and/or trading partner expected to distinguish the changed or new product from previous/current products? | Is there a regulatory/liability disclosure requirement to the consumer and/or trading partner? | Is there a substantial impact to the supply chain and trading partners (e.g., how the product is shipped, stored, received, or handled in the clinical setting)? |
| Pack/case quantity
| NO | YES | YES |
2.8.1 Pallet as a trade item
This rule applies when the pallet is a trade item and must be identified for ordering and invoicing purposes with a GTIN. In this case, GTIN allocation rules, including those for packaging hierarchy, apply.
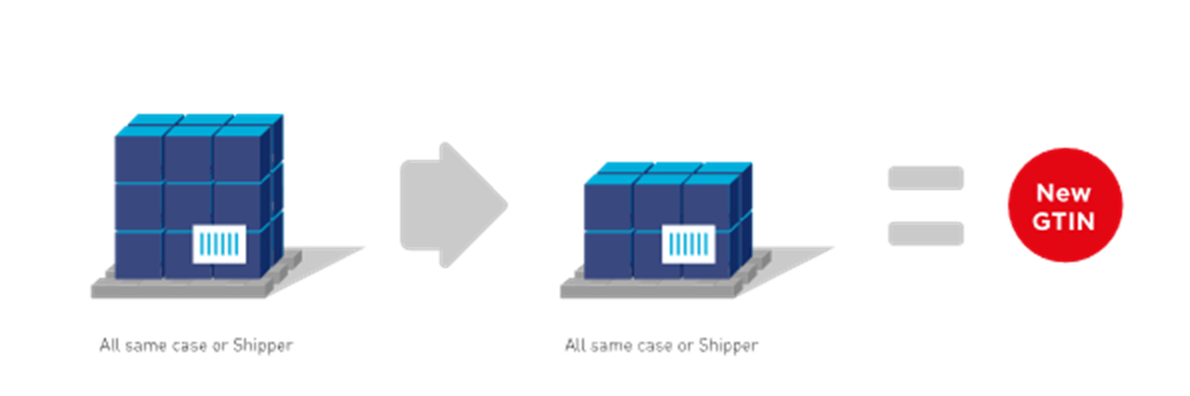
A change to the quantity of cases in a pre-defined pallet configuration requires assignment of a new GTIN. The pallet layout does not impact GTIN allocation of trade items packed in the cases on the pallet. Therefore, a change of a GTIN for a pallet does not necessitate a GTIN change to the lower levels of packaging.
Hierarchy levels of GTIN assignment:
■ A unique GTIN is assigned to each pallet configuration that contains different quantity of cases when the pallet is an orderable item.
Example business scenarios that require GTIN change:
Figure 2‑17 Additional or new pre-defined pallet configurations for ordering purposes require a different GTIN
Additional information:
■ Pallets require a GTIN only when they are a trade item (i.e. priced, ordered, or invoiced).
■ When a pallet is a logistics unit (e.g., shipment, transport, storage) it is identified with a Serial Shipping Container Code (SSCC).
■ Refer to the GS1 General Specifications for more information about logistics labelling and SSCCs.
A pre-defined assortment is defined as a pack of two or more different trade items that are combined and sold together as a single trade item.
A change, addition or replacement of one or more trade items included in a pre-defined assortment, requires assignment of a new GTIN.
Relevant guiding principles:
| GTIN Rule name | Is a consumer, patient, care provider and/or trading partner expected to distinguish the changed or new product from previous/current products? | Is there a regulatory/liability disclosure requirement to the consumer and/or trading partner? | Is there a substantial impact to the supply chain and trading partners (e.g., how the product is shipped, stored, received, or handled in the clinical setting)? |
| Pre-defined assortments | YES | YES | YES |
2.9.1 Kits
Kits are collections of non-homogeneous, separable components that are identified, purchased, and supplied as a single trade item for a specific clinical or commercial purpose.
There are two primary types of kits:
■ Finished product kit: Kits that are an assembly of only finished goods. Components are trade items, where each component is a trade item identified by a GTIN. Components do not need to be individually packaged; but are independently identified at the component packaging level, independent of the kit (e.g. may be sellable, identified and available for trade).
■ Manufactured kit: Kits that are completed or finished in the kitting process. At least one component of a manufactured kit is not a finished trade item and therefore is not identified with a GTIN.
Hierarchy levels of GTIN assignment:
The creator of the kit or kitter is responsible for allocating the GTIN to the kit.
■ The GTIN change occurs at the kit and all levels above.
The following GTIN change rules apply:
■ Adding a kit component requires a new GTIN. See Figure 2‑18.
■ Removal of a kit component requires a new GTIN. See Figure 2‑19.
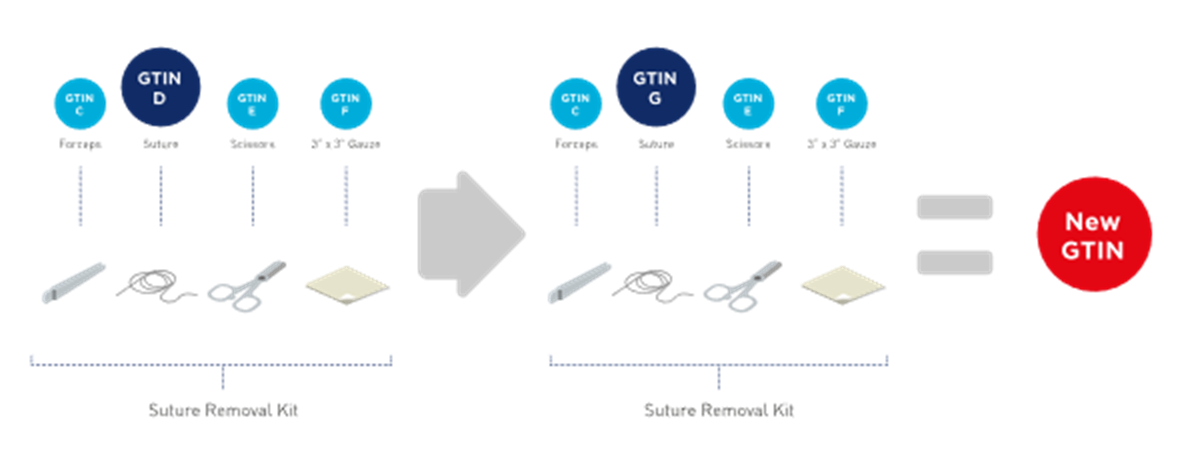
■ When kit components are identified by GTIN and/or brand owner item number, and that kit component is substituted, the kit GTIN Shall be changed. SeeFigure 2‑20.
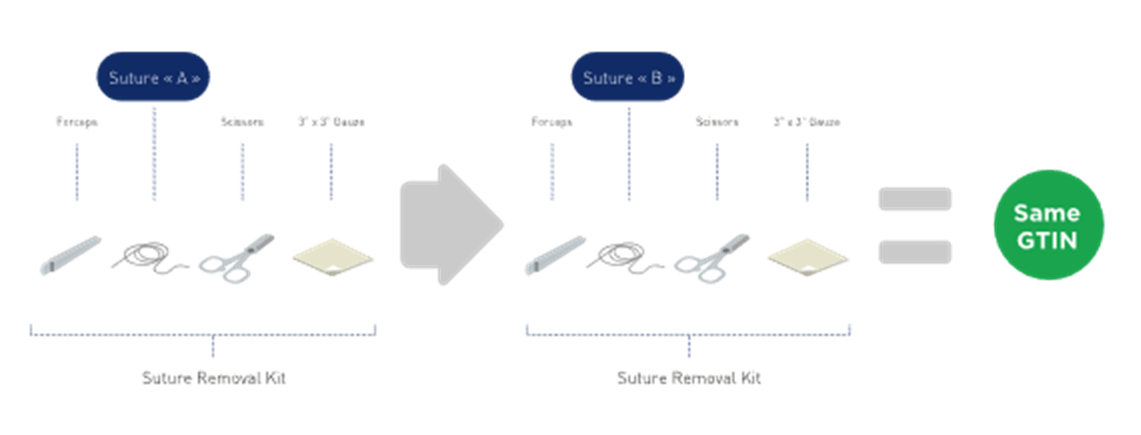
■ When kit components are listed by description only (i.e. no GTIN or brand owner item number), the kit manufacturer may substitute that kit component (maintaining form, fit, and function) without having to change the kit GTIN. See Figure 2‑21.
Example business scenarios that require GTIN change:
Figure 2‑18 Addition of a component to a kit
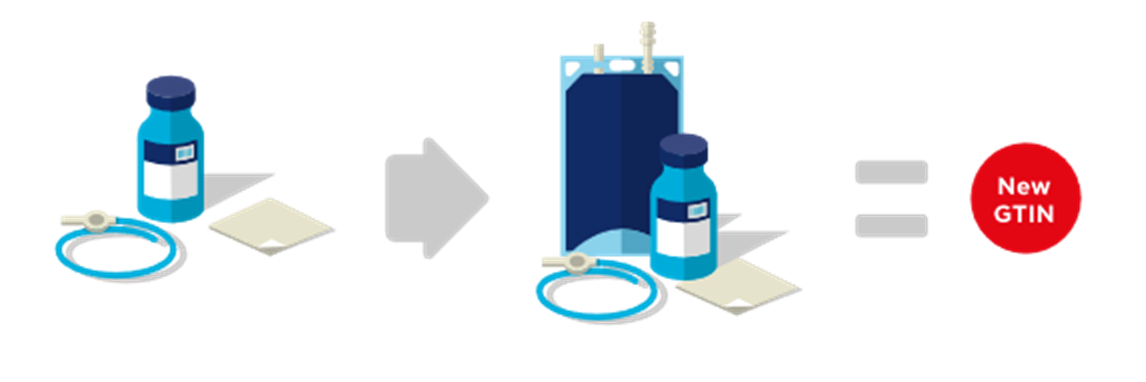
Figure 2‑19 Removal of a component from a kit
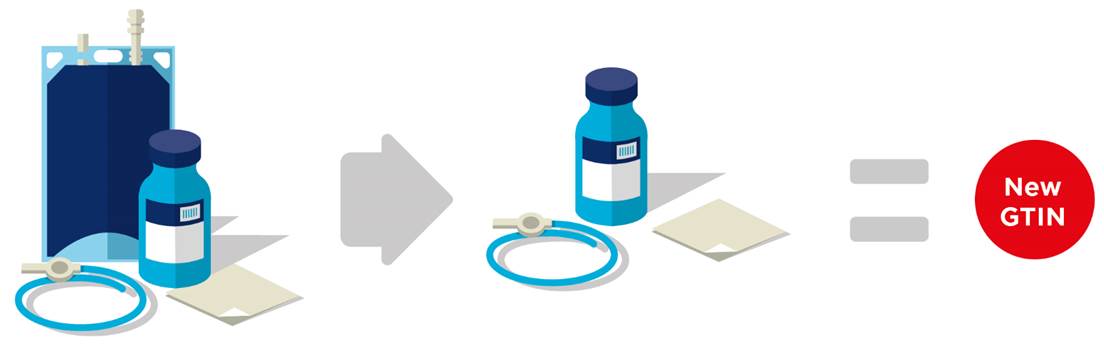
Figure 2‑20 Kit with specified components
Example business scenarios that do not require a GTIN change:
Figure 2‑21 Kit with unspecified components
Price on pack is defined as when the brand owner includes pre-pricing as part of the package graphics. This rule does not apply to prices marked on a price ticket, sticker, hangtag or anything that could be removed from the package or product.
Any addition, change or removal of a price marked directly on the product package (not recommended), requires assignment of a new GTIN.
Hierarchy levels of GTIN assignment:
■ The GTIN change occurs at the base unit level.
■ A unique GTIN is assigned at every existing level of the packaging hierarchy above the base unit level.
Example of business scenarios that require a GTIN change:
■ The pre-printed price on a package changes from €3 to €2.
■ A selling price of €8 is added to a product’s packaging graphic.
Additional information:
Pre-pricing is discouraged as a trade practice as it introduces complexity for trade item file maintenance through the supply chain. In addition, there is a danger that the price declaration to the consumer (on the pack) is different to the price charged (price in retailer(s) or healthcare system). However, pre-pricing can be a mandatory requirement from the regulatory authorities.
Relevant guiding principles:
| GTIN Rule Name | Is a care provider, consumer and/or trading partner expected to distinguish the changed or new product from previous/current products? | Is there a regulatory/liability disclosure requirement to the consumer and/or trading partner? | Is there a substantial impact to the supply chain (e.g., how the product is shipped, stored, received or handled in the clinical setting)? |
| Price in Pack | YES | YES | YES |
3 Clinical Trials
A clinical trial is conducted to investigate the efficacy and safety of treatments, interventions or tests – in preventing, managing or detecting disease or other medical conditions. Clinical trials can compare a new treatment to existing, test different combinations of existing treatments, or even look at other lifestyle factors and their impact on the patient’s well-being.
Clinical trials have product identification complexities not seen today in the commercial healthcare supply chain. The uniqueness of an investigational product, which in many cases is only for one patient means this has a need to be identified to the instance of the product. In blinded trials the blinded parties should not be able to see from the labelling whether the investigational product is a test article, comparator or placebo. Application of GS1 standards for clinical trials must take these complexities and industry needs into account.
In the event where the presentation of product changes, please refer to the Clinical Trial application standard.
■ For specific information on GTIN Allocation Rules refer to section 7 of the Identification of Investigational Products in Clinical Trials Application Standard.
■ For general information refer to the Identification of Investigational Products in Clinical Trials Application Standard.
4 Additional GTIN information
Global Trade Item Number
Global Trade Item Numbers (GTINs) can be used by a company to uniquely identify all of its trade items. GS1 defines trade items as any item (product or service) upon which there is a need to retrieve predefined information and that may be priced, ordered, or invoiced at any point in any supply chain. For more information on GTIN refer to the GS1 General Specifications.
Structure of a GTIN
Companies can license a GS1 Company Prefix from a GS1 Member Organization that comes with full documentation on how to allocate GTINs to their products.
GTINs should be treated as non-significant numbers. This means that they should always be recorded and processed in their entirety; no part of the number relates to any classification or conveys any information.
GS1 Company Prefixes are licensed by GS1 Member Organisations to a user company to entitle that user company to create any of the GS1 identification keys, such as GTIN, SSCC, or GIAI.
![]() Note: While the GS1 Company Prefix can be used to determine which GS1 Member organisation allocated the prefix, it cannot be used to determine where an item was produced or distributed.
Note: While the GS1 Company Prefix can be used to determine which GS1 Member organisation allocated the prefix, it cannot be used to determine where an item was produced or distributed.
U.P.C. Company Prefix
A U.P.C. Company Prefix is derived from a GS1 Company Prefix that starts with zero (‘0’) by removing that leading zero. A U.P.C. Company Prefix SHALL only be used to construct 12-digit trade item identifiers (e.g. GTIN-12). When a leading zero is added to a U.P.C. Company Prefix it becomes a GS1 Company Prefix that may be used to issue all other GS1 identification keys.
![]() Note: For example, the 6-digit U.P.C. Company Prefix 614141 is derived from the 7-digit GS1 Company Prefix 0614141.
Note: For example, the 6-digit U.P.C. Company Prefix 614141 is derived from the 7-digit GS1 Company Prefix 0614141.
The item reference is a component of the Global Trade Item Number (GTIN) assigned by the organisation to whom the GS1 Company Prefix or U.P.C. Company Prefix has been licensed to create a unique GTIN and is a non-significant number, which means that the individual digits in the number do not relate to any classification or convey any specific information. The simplest way to allocate the item references is sequentially, that is 000, 001, 002, 003, etc.
The check digit is the last digit. It is calculated from all other digits in the GTIN.
The figure below shows the four GTIN structures and how they should be stored in a database. The GTINs should be right justified and zero filled on the left.
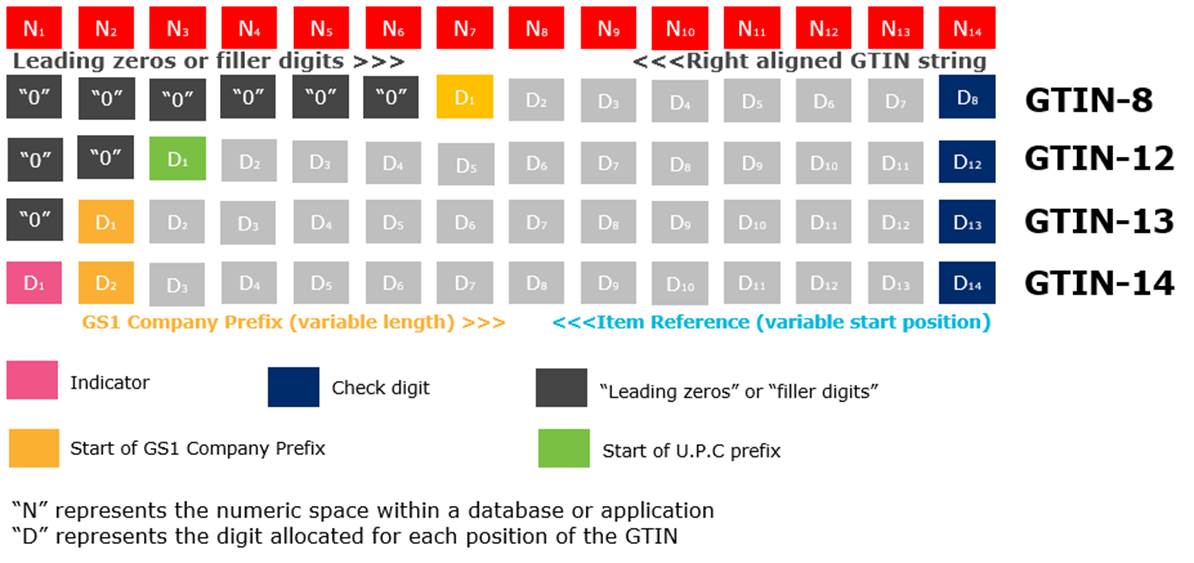
![]() Note 1: When any of these GTINs are encoded in a data carrier that must encode a fixed-length data string of 14-digits, the GTINs when less than 14-digits in length must be prefixed by padded zeros that simply act as filler digits. Adding fill zeros does not change a GTIN-8, 12 or 13 into a GTIN-14. However, the “filler” zeros are not encoded in EAN-8, U.P.C-A or EAN-13 barcodes.
Note 1: When any of these GTINs are encoded in a data carrier that must encode a fixed-length data string of 14-digits, the GTINs when less than 14-digits in length must be prefixed by padded zeros that simply act as filler digits. Adding fill zeros does not change a GTIN-8, 12 or 13 into a GTIN-14. However, the “filler” zeros are not encoded in EAN-8, U.P.C-A or EAN-13 barcodes.
![]() Note: 2: The 14-digit GTIN format is used in business transactions, especially for eCommerce (e.g., electronic orders, invoices, price catalogues, etc.) and in the Global Data Synchronisation Network (GDSN).
Note: 2: The 14-digit GTIN format is used in business transactions, especially for eCommerce (e.g., electronic orders, invoices, price catalogues, etc.) and in the Global Data Synchronisation Network (GDSN).
Indicator
The indicator is only used in the GTIN-14 data structure. It takes the value 1 to 8 (see Note 1 below) and is used for lower or higher packaging levels. The simplest way to allocate the indicator is sequentially that is 1, 2, 3… to each trade item grouping.
A uniform grouping of trade items is an orderable grouping of identical trade items. The brand owner has the option of either assigning a unique GTIN-13 or GTIN-12 to each grouping or assigning a unique GTIN-14 with an indicator digit value of 1 to 8. These GTIN-14s incorporate the GTIN of the trade item (less its check digit) contained in each grouping. The check digit for each GTIN-14 is then recalculated. Due to the value options of 1 to 8, eight, separate, unique GTIN-14s can be created from a single GTIN-13 or GTIN-12.
The indicator digits have no meaning. The digits do not have to be used in sequential order and some may not be used at all. The GTIN-14 structure for standard trade item groupings creates extra numbering capacity. The indicator digit assigned as required by the company that constructs the GTIN.
![]() Note 1: The indicator value 9 is reserved for variable measure items. Variable measure: If the measure of items contained in a case, is not pre-defined then the trade item is of variable measure. Some of the attributes related to this variable measure trade item, such as the count of units contained and the weight for example, are not pre-defined but will be known only when the product is manufactured. It needs to be decided upfront if an item is a fixed measure one with pre-defined essential attributes or a variable measure one, where one measure such as the count of units contained, is not pre-defined but is specific to each instance. In such cases a GTIN-14 with Indicator 9 and Application Identifier (30) may be used. For more information refer to the Variable count of items: AI (30) section of the GS1 General Specifications. Additional information on variable measure is found is the following sections of the GS1 General Specification: Variable measure trade items scanned in general distribution and Variable measure trade items scanned at retail Point of Sale.
Note 1: The indicator value 9 is reserved for variable measure items. Variable measure: If the measure of items contained in a case, is not pre-defined then the trade item is of variable measure. Some of the attributes related to this variable measure trade item, such as the count of units contained and the weight for example, are not pre-defined but will be known only when the product is manufactured. It needs to be decided upfront if an item is a fixed measure one with pre-defined essential attributes or a variable measure one, where one measure such as the count of units contained, is not pre-defined but is specific to each instance. In such cases a GTIN-14 with Indicator 9 and Application Identifier (30) may be used. For more information refer to the Variable count of items: AI (30) section of the GS1 General Specifications. Additional information on variable measure is found is the following sections of the GS1 General Specification: Variable measure trade items scanned in general distribution and Variable measure trade items scanned at retail Point of Sale.
![]() Note 2: Some scanners at retail point-of-sale may not be capable of reading and interpreting barcode symbologies other than EAN/UPC, which cannot encode a GTIN-14.
Note 2: Some scanners at retail point-of-sale may not be capable of reading and interpreting barcode symbologies other than EAN/UPC, which cannot encode a GTIN-14.
![]() Note 3: See GS1 General Specifications GTIN sections for further information.
Note 3: See GS1 General Specifications GTIN sections for further information.
![]() Note 4: For more information on how to construct a GTIN refer to How to create a GTIN on the GS1 website or contact your local GS1 Member Organisation.
Note 4: For more information on how to construct a GTIN refer to How to create a GTIN on the GS1 website or contact your local GS1 Member Organisation.
5 Glossary of terms
For latest definition of terms refer to www.gs1.org/glossary.
| Term | Definition |
| Application identifier (AI) | The field of two or more characters at the beginning of a GS1 Element String that uniquely defines its format and meaning. |
| Assay | An assay is an investigative (analytic) procedure in laboratory medicine, pharmacology, environmental biology and molecular biology for qualitatively assessing or quantitatively measuring the presence, amount, or functional activity of a target entity (the analyte) |
| barrier pack | A type of package shielding the contents from contact with external substances or other external influences. Depending on the material and the packaging process it may provide protection against e.g. light, moisture or microorganisms, i.e. preserve the sterility of the contents. |
| blister pack | A type of package in which the material (frequently plastic or metal foil) is formed to one or more blisters, each usually containing one unit of the product. The open bases of the blister cells are usually sealed by affixing a layer of plastic or metal foil or of paper which will be perforated when a cell is accessed, thus making the manipulation obvious (tamper evidence). |
| brand owner | The organisation that owns the specifications of a trade item, regardless of where and by whom it is manufactured. The brand owner is normally responsible for the management of the Global Trade Item Number (GTIN). |
| breather packaging/pouch | A package of layer of fibrous material. Breather package for surgical elements such as sutures or suture-needle assemblies having a layer of fibrous material and a layer of plastic material forming a pocket between. |
| check digit | A final digit calculated from the other digits of some GS1 identification keys. This digit is used to check that the data has been correctly composed. |
| caplet | A coated tablet for oral medication. |
| Co-branding | The act of applying an additional recognisable brand (secondary brand), logo, trademark or registration mark to a product label or package, where it co-exists with the primary brand. This would normally be performed under contractual agreement with the original brand owner. |
| direct part marking | Direct part marking refers to the process of marking a symbol on an item using an intrusive or non-intrusive method. |
| dosage | The quantity per application and the frequency of application of a drug. |
| each/base unit | In a packaging hierarchy of trade items, the Base Unit or Each denotes the retail consumer trade item level. The term each refers to the lowest traded packaging level. This level might contain more than one single unit/unit of use. |
| Electronic Product Code (EPC) | An identification scheme for universally identifying physical objects (e.g., trade items, assets, and locations) via RFID tags and other means. The standardised EPC data consists of an EPC (or EPC Identifier) that uniquely identifies an individual object, as well as an optional filter value when judged to be necessary to enable effective and efficient reading of the EPC tags. |
| element string | The combination of a GS1 Application Identifier and a GS1 Application Identifier data field. |
| equivalent | A product which can be substituted for the trade item based on supplier- defined functional equivalence to the trade item. |
| formulation | Definition of the combination of different chemical substances, including the active ingredients, constituting a final medicinal product. |
| Form, fit or function | A change in the product’s specifications or design that changes the intended purpose / use and needs to be communicated to the customer. |
| GS1 Company Prefix (GCP) | A unique string of four to twelve digits used to issue GS1 identification keys. The first digits are a valid GS1 Prefix and the length must be at least one longer than the length of the GS1 Prefix. The GS1 Company Prefix is issued by a GS1 Member Organisation. As the GS1 Company Prefix varies in length, the issuance of a GS1 Company Prefix excludes all longer strings that start with the same digits from being issued as GS1 Company Prefixes. See also U.P.C Company Prefix. |
| GS1 General Specifications | Defines the GS1 system data and application standards related to the marking and automatic identification of trade items, locations, logistic units, assets, and more using barcode, RFID, and GS1 identification keys. |
| GS1 Global Office | Based in Brussels, Belgium, and Princeton, USA, is an organisation of GS1 Member Organisations that manages the GS1 system. |
| GS1 Member Organisation (GS1 MO) | A member of GS1 that is responsible for administering the GS1 system in its country (or assigned area). This task includes, but is not restricted to, ensuring user companies make correct use of the GS1 system, have access to education, training, promotion and implementation support and have access to play an active role in GSMP. |
| GS1 system | The specifications, standards, and guidelines administered by GS1. |
| Global Trade Item Number® (GTIN®) | The GS1 identification key used to identify trade items. The key comprises a GS1 Company Prefix, an item reference and check digit. |
| Global Data Synchronisation Network (GDSN) | The GS1 Global Data Synchronisation Network® (GDSN®) is a network of interoperable data pools enabling collaborating users to securely synchronise master data based on GS1 standards. |
| Graphic user interface (GUI) | Graphical user interface - is a form of user interface that allows users to interact with electronic devices through graphical icons and audio indicator such as primary notation, instead of text-based user interfaces, typed command labels or text navigation. |
| indicator | A digit from 1 to 9 in the leftmost position of the GTIN-14. |
| item reference | A component of the Global Trade Item Number (GTIN) assigned by the brand owner to create a unique GTIN. |
| HRI | Human readable interpretation (HRI) is the information below, beside or above a barcode or tag which is encoded in the barcode or tag and represents the same characters as carried in the barcode or tag. Refer to the GS1 General Specifications, section Human readable interpretation (HRI) rules for further information. |
| kit | A collection of different regulated healthcare items assembled for use in a single therapy. |
| Kitter | The brand owner that defines the kit contents, specifications and labelling. The kitter may or may not assemble kits and may engage a third party to produce the finished trade items. |
| Level below the each | The lowest hierarchy level of trade items within the GS1 System is traditionally referred to as the “each” level. The “each” level trade item may contain more than one unit of use. In this case there may be the need to identify levels below the ‘each’ down to the single unit or unit of use. In the Healthcare industry, there can be a “smaller” or “lower” unit, which will be scanned at the “Healthcare Point of Care, commonly referred to as the “Level Below the Each” |
| medical device | Any instrument, apparatus, implement, machine, appliance, implant, in vitro reagent or calibrator, software, material or other similar or related article, intended by the manufacturer to be used, alone or in combination, for human beings for any medical purpose. |
| Non-HRI | Non-HRI text is all other text on package, label or item. Refer to the GS1 General Specifications, section Human readable interpretation (HRI) rules for further information. |
| Pharmaceutical product | A pharmaceutical is any kind of drug used for medicinal purposes, like cough syrup or sleeping pills. |
| Primary brand | The primary brand is the brand most recognisable by the care provider or patient, as determined by the brand owner, and can be expressed as a logo, registration mark or trademark. |
| primary package | The innermost layer of packaging, i.e. the layer closest to the product (pill, implant, instrument, etc.). |
| Regulated Healthcare Trade Item | A regulated healthcare trade item are pharmaceuticals or medical devices that are sold or dispensed in a controlled environment (e.g. retail pharmacy, hospital pharmacy). |
| Rx (medical prescription product)
| A drug or medicinal specialty that requires a medical prescription or direct medical intervention. Typical examples include, medicated bandages, pain medication, injectables etc. and can normally only be obtained with a prescription from an appropriate health care practitioner. |
| seal inner through outer (SITO) | A type of barrier package consisting of two layers. In a special production process the inner layer (frequently a foil pouch) initially stays open for sterilisation of the contents and then is sealed through the outer layer, which was closed before and stays intact throughout. |
| secondary package | The layer of packaging surrounding the primary package. May be used for purpose of presentation and branding or for additional mechanical protection the primary package might not be able to provide. May contain one or more primary packages. |
| single unit package/blister
| A healthcare primary package that contains one discrete pharmaceutical dosage form, i.e. a tablet, a certain volume of a liquid or that is the immediate package for a medical device like a syringe. Several single units may be attached to each other but are easy to separate through a perforation. |
| strength | Amount of active ingredient(s) in a drug. |
| trade item: | Any item (product or service) upon which there is a need to retrieve predefined information and that may be priced, or ordered, or invoiced at any point in any supply chain |
| U.P.C. Company Prefix | A GS1 Company Prefix starting with a zero (‘0’) becomes a U.P.C. Company Prefix by removing the leading zero. A U.P.C. Company Prefix is used to issue GTIN-12. |
Change log and contributors
Contributors
| Name | Organisation |
| Mark Hoyle (Chair) | Teleflex Inc. |
| Scott Mooney (Chair) | McKesson |
| Hajo Reissmann (Chair) | Universitaetsklinikum Schleswig-Holstein |
| Stacey Henning | Cardinal Health |
| Feargal Mc Groarty | St. James's Hospital |
| Stefan Artlich | Bayer AG - Division Pharma |
| Vincent Auger | CEMO |
| Carey Barlett | Teleflex Inc. |
| Odile Baud | SANOFI |
| Robert Bernardo | Pfizer |
| Dennis Black | BD |
| Lynn Carothers | Teleflex Inc. |
| Christine Chang | 3M Healthcare |
| Trey Davis | Alcon Labs |
| Charlene Ekeren | 3M Healthcare |
| Zachary Garrison | Abbott |
| Paula Giovannetti | Nestlé HealthScience |
| Shauntell Harper | Smith & Nephew |
| Nils Haugen | 3M Healthcare |
| Michael Hoefling | Boehringer Ingelheim Pharma GmbH & Co.KG |
| Wendy Jackson | Boston Scientific Corp |
| Matthias Kallmeyer | Boehringer Ingelheim Pharma GmbH & Co.KG |
| Sébastien Langlois-Berthelot | F. Hoffmann-La Roche Ltd. |
| Sonja Lukic | Fresenius Kabi AG |
| Marina Madokoro | Johnson & Johnson |
| Patrick Main | Cook Medical Inc. |
| Michelle Oliveira | Boston Scientific Corp |
| Tatjana Pathare | F. Hoffmann-La Roche Ltd. |
| Nicole Sampson | 3M Healthcare |
| Pauline Senegas | Laboratoire Pierre Fabre dermo -cosmétique |
| April Anne Sese | Johnson & Johnson |
| Angela Silvestri | Stryker |
| Brad Steger | Zimmer Biomet US |
| John Terwilliger | Abbott |
| Olga van Grol | Boston Scientific Corp |
| Nikola Cathcart-Sievert | Wal-Mart Stores, Inc. |
| Tracy Scott | Wal-Mart Stores, Inc. |
| Melissa Banning | TraceLink |
| Jay Crowley | US Data Management, LLC (USDM) |
| Dilip Daswani | Qliktag Software (formally Zeebric LLC) |
| Christophe Devins | Adents High-Tech International |
| James Grant | Health Support Queensland |
| W. Carl Henshaw | Vizient, Inc. |
| Ibrahim Hoxha | HOXHA |
| Roula Karam | Antares Vision |
| Nancy LeMaster | Nancy J LeMaster Consulting |
| Paola Morales | Logyca |
| Brigitte Naftalin | Adents High-Tech International |
| Susan Ramonat | Spiritus Partners |
| Vincent Robolt | Essilor |
| Michael Sarachman | US Data Management, LLC (USDM) |
| Julien Taburel | Adents High-Tech International |
| Elizabeth Waldorf | TraceLink |
| Majd Haddaji | EDICOM |
| Shreenidhi Bharadwaj | Syndigo |
| Scott Brown | 1WorldSync, Inc. |
| J.D. Kern | Syndigo |
| Mattthew Muldoon | Syndigo |
| Oleg Vinichenko | SKB Kontur |
| Steven Simske | Colorado State University |
| Sean Lockhead | USAID GHSC-PSM |
| Ryan Mavin | ACT Health |
| Samuel Oh | USAID GHSC-PSM |
| Martin Kairu | GS1 South Africa |
| Mirva Alatyppö | GS1 Finland |
| Mónica Arango | GS1 Colombia |
| Andrea Arozamena | GS1 Mexico |
| Madalena Centeno | GS1 Portugal |
| Jiraporn Chalermjirarat | GS1 Thailand |
| Shawn Chen | GS1 Thailand |
| Mignone Cheng | GS1 Hong Kong, China |
| Pavla Cihlarova | GS1 Czech Republic |
| Luiz Costa | GS1 Brasil |
| Ferran Domenech Fuste | GS1 Spain |
| Angela Fernandez | GS1 US |
| Jesper Kervin Franke | GS1 Denmark |
| Stefan Gathmann | GS1 Ireland |
| Anna Gawronska | GS1 Poland |
| Beth Wells | GS1 US |
| Nicole Golestani | GS1 Canada |
| Marija Groznik Stankovic | GS1 Slovenia |
| Rami Habbal | GS1 UAE |
| Michaela Hähn | GS1 Germany |
| Christian Hay | GS1 Switzerland |
| Christine Horvath-Hanko | GS1 Hungary |
| Yoshihiko Iwasaki | GS1 Japan |
| Fiona (Zhitao) Jia | GS1 China |
| iliada karali | GS1 Association Greece |
| Kimmo Keravuori | GS1 Finland |
| Anna Klapper | GS1 Germany |
| Catherine Koetz | GS1 Australia |
| Cihan Korucu | GS1 Turkey |
| Jenni Krohn | GS1 Finland |
| Camille Labeaune | GS1 France |
| Ildikó Lieber | GS1 Hungary |
| Xiaoyan Liu | GS1 China |
| Osiris López Rojas | GS1 Mexico |
| Marisa Lu | GS1 Chinese Taipei |
| Ilka Machemer | GS1 Germany |
| Fumi Maekawa | GS1 Japan |
| Valerie Marchand | GS1 France |
| Daniel Mueller-Sauter | GS1 Switzerland |
| Zubair Nazir | GS1 Canada |
| Giada Necci | GS1 Italy |
| Alice Nguyen | GS1 Vietnam |
| Leonel Pava | GS1 Colombia |
| Sarina Pielaat | GS1 Netherlands |
| Aruna Ravikumar | GS1 Australia |
| Paul Reid | GS1 UK |
| Sylvia Reingardt | GS1 Germany |
| Marcia Saba | GS1 Brasil |
| Sue Schmid | GS1 Australia |
| Eugen Sehorz | GS1 Austria |
| Julian Sin | GS1 Hong Kong, China |
| Mig Smith | GS1 UK |
| Hiromitsu Takai | GS1 Japan |
| Koichi Uemura | GS1 Japan |
| Vivian Underwood | GS1 US |
| Ricardo Verza Amaral Melo | GS1 Brasil |
| Hanna Walczak | GS1 Poland |
| Amber Walls | GS1 US |
| Roland Weibel | GS1 Switzerland |
| Beth Wells | GS1 US |
| Brian Wells | GS1 US |
| Connie Wong | GS1 Canada |
| Pete Alvarez | GS1 Global Office |
| Henri Barthel | GS1 Global Office |
| Chuck Biss | GS1 Global Office |
| David Buckley | GS1 Global Office |
| Steven Keddie | GS1 Global Office |
| Ulrike Kreysa | GS1 Global Office |
| Geraldine Lissalde-Bonnet | GS1 Global Office |
| Neil Piper | GS1 Global Office |
| Laure Pontis | GS1 Global Office |
| Greg Rowe | GS1 Global Office |
Log of Changes
| Release | Date of Change | Changed By | Summary of Change |
| 9.0.1 | July 2015 | Valerie Hoste | Applied new GS1 branding and errata fix to add figure heading 5-11 (example of a medical device) |
| 9.0.2 | Dec 2015 | David Buckley | Errata fix, wrong cross-references in section 5.1.8 and fixed corrupted figure cross-references |
| 10.0 | Jun 2020 | Pete Alvarez | Work Request 18-294. Conducted complete review of the entire document, clarified and updated content throughout. Harmonised with the GTIN Management Standard as appropriate including matching table of content for consistency and cross reference. Added information for Clinical Trials. |
Useful links:
* PDF version of this GS1 Healthcare GTIN Allocation Rules Standard
* Decision support tool https://www.gs1.org/1/hcgtinrules/en/decision-support
* translations of this standard will be posted on https://www.gs1.org/1/gtinrules/healthcare